Answer:
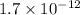 moles of carbon
moles of carbon
Step-by-step explanation:
1 trillion =
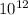
Avogadro number :It is the number of particles that are present in 1 mole of substance.
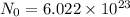
So,
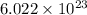 atoms = 1 mole carbon
atoms = 1 mole carbon
1 atom =
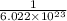 mole
mole
1 trillion atom =
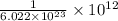 mole
mole
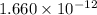 mol
mol
Two significant figure
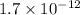 moles of carbon
moles of carbon