Answer:
The answer to your question is given after the questions so I just explain how to get it.
Step-by-step explanation:
a)
Get the molecular weight of Phosphoric acid
H₃PO₄ = (3 x 1) + (31 x 1) + (16 x 4)
= 3 + 31 + 64
= 98 g
98 g ----------------- 1 mol
0.045 g --------------- x
x = (0.045 x 1) / 98
x = 0.045 / 98
x = 0.00046 moles or 4.6 x 10 ⁻⁴
b)
Molarity =
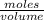
Molarity =
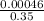
Molarity = 0.0013 or 1.31 x 10⁻³
c)
Formula C₁V₁ = C₂V₂
V₁ = C₂V₂ / C₁
Substitution
V₁ = (0.0013)(1) / 0.01
Simplification and result
V₁ = 0.0013 / 0.1
V₁ = 0.13 l = 130 ml