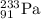Answer:
Step-by-step explanation:
In a nuclear reaction, the total mass and total atomic number remains the same. It is mentioned that the a alpha particle,
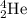 is being emitted.
is being emitted.
For the given fission reaction:
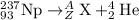
To calculate A:
Total mass on reactant side = total mass on product side
237 = A + 4
A = 233
To calculate Z:
Total atomic number on reactant side = total atomic number on product side
93 = Z + 2
Z = 91
Hence, the isotopic symbol of unknown element is