Answer:
The lowest osmotic pressure is produced by the solution with the lowest concentration of solute.
Step-by-step explanation:
This relation between the osmotic pressure and the concentration of solute was determined by Jacobus Van 't Hoff and follows the formula:
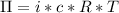
Where:
 is the osmotic pressure
is the osmotic pressure
- i is the Van 't Hoff index
- c is the molar concentration of solute
- R is the ideal gas constant
- T is the temperature
Given that R is a constant and that i and T are the same for all the solutions, the solution with the lowest c will have the lowest osmotic pressure.