Answer:
Potassium Dichromate(K2Cr2O7) is oxidising agent and will oxidise Hydrogen sulfide(H2S) to Sulphur(S).
The orange color of Potassium dichromate is changed to green due formation of Chromium sulphate.
Step-by-step explanation:
The balance chemical equation for the reaction is :
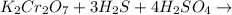
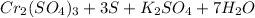
K2Cr2O7 = It is the commonly used inorganic reagent used for its oxidizing properties .It is crystalline , bright orange coloured.
In this reaction it oxidise Hydrogen sulfide to sulfate .
Hydrogen Sulfide = It has Formula H2S . It is a colourless gas .
It is foul smelling and act as reducing agent .
In this reaction H2S reduces Potassium dichromate(Cr = +6) to Chromium Sulfate (Cr = +3)