Answer:
136.67 g of Na3PO4 is required to create 100 gram of NaOH.
Step-by-step explanation:
The balanced equation:
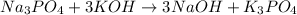
1 mole Na3PO4 = 164 g/mole (Molar mass)
1 mole NaOH = 40 g/mole (Molar mass)
Now,
1 mole of Na3PO4 produce = 3 mole of NaOH
164 g/mol of Na3PO4 produce = 3(40) g/mol of NaOH
or
120 g/mol of NaOH is produced from = 164 g/mol of Na3PO4
1 g/mol of NaOH is produced from =
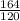
100 grams of NaOH is produced from =
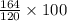 gram of Na3PO4
gram of Na3PO4
calculate,
= 136.67 g