Answer:
1.417
Step-by-step explanation:
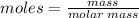
The molar mass or the neutron number can be found from the periodic table.
There are two elements in H2O
- Hydrogen, molar mass 1
- Oxygen, molar mass 16
To find the total molar mass add 2 hydrogens and one oxygen mass. So 2(1)+16=18