Answer: The phase change process in which solids gets converted to gases is sublimation.
Step-by-step explanation:
For the given options:
Option a: Condensation
It is a type of process in which phase change occurs from gaseous state to liquid state at constant temperature.
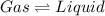
Option b: Melting
It is a type of process in which phase change occurs from solid state to liquid state at constant temperature.
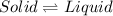
Option c: Sublimation
It is a type of process in which phase change occurs from solid state to gaseous state without passing through the liquid state at constant temperature.
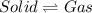
Option d: Deposition
It is a type of process in which phase change occurs from gaseous state to solid state without passing through the liquid state at constant temperature.
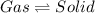
Hence, the phase change process in which solids gets converted to gases is sublimation.