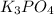The question is incomplete, here is a complete question.
Which aqueous solution would have the lowest vapor pressure at 25°c.
A) 1 M NaCl
B) 1 M
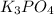
C) 1 M
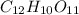
D) 1 M
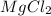
E) 1 M
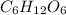
Answer : The correct option is, (B) 1 M
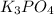
Explanation :
According to the relative lowering of vapor pressure, the vapor pressure of a component at a given temperature is equal to the mole fraction of that component of the solution multiplied by the vapor pressure of that component in the pure state.
1 M means that the 1 moles of solute present in 1 liter of solution.
Formula used :
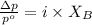
where,
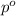 = vapor pressure of the pure component (water)
= vapor pressure of the pure component (water)
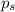 = vapor pressure of the solution
= vapor pressure of the solution
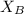 = mole fraction of solute
= mole fraction of solute
i = Van't Hoff factor
As we know that the vapor pressure depends on the mole fraction of solute and the Van't Hoff factor.
So, the greater the number of particles of solute dissolved the lower the resultant vapor pressure.
(a) The dissociation of 1.0 M
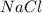 will be,
will be,
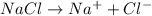
So, Van't Hoff factor = Number of solute particles =
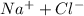 = 1 + 1 = 2
= 1 + 1 = 2
(b) The dissociation of 1 M
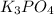 will be,
will be,
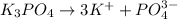
So, Van't Hoff factor = Number of solute particles =
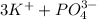 = 3 + 1 = 4
= 3 + 1 = 4
(c) The dissociation of 1 M
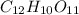 is not possible because it is a non-electrolyte solute. So, the Van't Hoff factor will be, 1.
is not possible because it is a non-electrolyte solute. So, the Van't Hoff factor will be, 1.
(d) The dissociation of 1.0 M
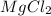 will be,
will be,
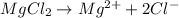
So, Van't Hoff factor = Number of solute particles =
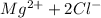 = 1 + 2 = 3
= 1 + 2 = 3
(e) The dissociation of 1 M
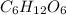 is not possible because it is a non-electrolyte solute. So, the Van't Hoff factor will be, 1.
is not possible because it is a non-electrolyte solute. So, the Van't Hoff factor will be, 1.
From this we conclude that, 1 M
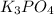 has the highest Van't Hoff factor which means that the solution will exhibit the lowest vapor pressure.
has the highest Van't Hoff factor which means that the solution will exhibit the lowest vapor pressure.
Hence, the correct option is, (B) 1 M