The given question is incorrect, here is a complete question.
Given the equation representing a reaction:
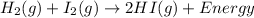
Which statement describes the energy changes that occur in this reaction?
(1) Energy is absorbed as bonds are formed, only.
(2) Energy is released as bonds are broken, only.
(3) Energy is absorbed as bonds are broken, and energy is released as bonds are formed.
(4) Energy is absorbed as bonds are formed, and energy is released as bonds are broken.
Answer : The correct option is, (3) Energy is absorbed as bonds are broken, and energy is released as bonds are formed.
Explanation :
Bond energy : It is defined as the amount of energy required to break a molecules into its component atoms.
At the time of bond formation, the energy is released and at the time of bond breaking, the energy is absorbed.
In the chemical reaction, when the energy is shown in product side that means the energy is released.
In the chemical reaction, when the energy is shown in reactant side that means the energy is absorbed.
Hence, the correct option is, (3)