Step-by-step explanation:
It is known that ionic compounds are the compounds formed by transfer of electrons from one atom to another. Ionic compounds have high boiling point because their combining atoms contain opposite charges due to which strong forces of interaction exists between them.
A metallic bond is formed due to mobile valence electrons shared by positive nuclei in a metallic crystal.
When a bond is formed by sharing of electrons between the combining atoms then the compound formed is known as covalent compound. In these compounds there exists weak forces due to sharing of electrons and therefore, they have low boiling point.
Hydrogen bonding is defined as a bonding which exists between a hydrogen atom and an electronegative atom like O, N and F.
Therefore, out of the given options KI is an ionic compound and it will have high boiling point due to the presence of strong interactive forces between hydrogen and fluoride ions.
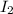 being a covalent compound has low boiling point.
being a covalent compound has low boiling point.
The compound HF has hydrogen bonding and its boiling point is around
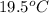 . Whereas HI has low boiling point due to the large difference in the size of H and I. Magnesium being a metallic solid will have boiling point more that a covalent compound but less than an ionic compound.
. Whereas HI has low boiling point due to the large difference in the size of H and I. Magnesium being a metallic solid will have boiling point more that a covalent compound but less than an ionic compound.
Thus, we can conclude that given species are arranged from highest boiling point (top) to lowest boiling point (bottom) as follows.
KI > Mg >
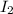 > HF > HI
> HF > HI