Answer:
4 moles, 160 g
Step-by-step explanation:
The formula for the calculation of moles is shown below:
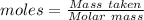
For
 :-
:-
Mass of
 = 196 g
= 196 g
Molar mass of
 = 98 g/mol
= 98 g/mol
The formula for the calculation of moles is shown below:
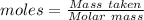
Thus,
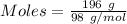
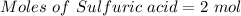
According to the given reaction:
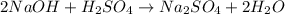
1 mole of sulfuric acid reacts with 2 moles of NaOH
So,
2 moles of sulfuric acid reacts with 2*2 moles of NaOH
Moles of NaOH must react = 4 moles
Molar mass of NaOH = 40 g/mol
Mass = Moles*molar mass =
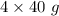 = 160 g
= 160 g