Answer:
There are 899.88 moles.
Step-by-step explanation:
To solve this problem we'll need to use Avogadro's number, which tells us that in one mole of a species, there are 6.023x10²³ atoms (or molecules).
With the above information in mind we can calculate the moles in 5.42x10²⁶ atoms of calcium:
- 5.42x10²⁶ atoms *
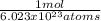 = 899.88 moles
= 899.88 moles