Answer:
The count rate at the end of four days will be 7.5 counts per minute.
Step-by-step explanation:
First it is important to know that half-life is the time to a piece of radioactive material to decay 50%. So if we know we start with 120 counts per minute and we already know the half-life of the isotope is 1 hour we expect that past 1 hour the material decays 50% (it's halved) so we will count 60 counts per minute, now if we wait another hour 60 counts will decay in to 30 counts per minute and so on. That should be translate to a math equation as:
final counts = initial counts *
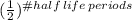
After 4 days we have 4 half-life periods passed so:
final counts= 120 counts per minute *
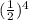
final material = 7.5 counts per minute