Answer:
b. Larger for atoms of high atomic number.
Step-by-step explanation:
The atomic number of a chemical element is the number of protons found in the nucleus of every atom of that element.
The electrical force between an inner electron and the nucleus of an atom is described by the Coulomb law
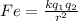
where k: Coulomb constant
q1 and q2 are the electric charges that interact (in our case electron and protons)
r: Distance between electron and each proton
Then, larger number of protons in the nucleus, more the electrical force will be.
The answer is:
b. Larger for atoms of high atomic number.