Answer:
26. Mass of sugar = 100 g
27. a).m/m % = 8.67 %
b).m/m% = 20%
28.a). Mass of sugar = 12.5 g
b).Mass of sugar = 12.5 g
c).Mass of sugar = 30 g
Step-by-step explanation:
26. Use formula:
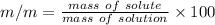
m/m = 20%
mass of solution = 500 g
mass of Solute = mass of sugar = ?
insert in the formula,
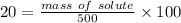
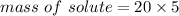
mass of solute = 100 g
27. a)
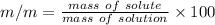
mass of Barium hydroxide(solute) = 13 g
mass of solution = 150 g
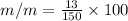
m/m % = 8.67 %
b).
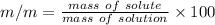
mass of Glucose (solute) = 50 g
mass of solution = 250 g
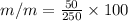
m/m% = 20%
28. a)
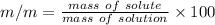
m/m% = 5
mass of solution = 250 g
mass of solute = ?
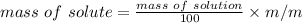
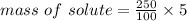
mass of sugar = 12.5 g
b).
m/m% = 2.5%
mass of solution = 500 g
mass of solute = ?
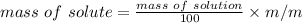
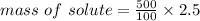
mass of sugar = 12.5 g
c).
m/m% = 3 %
mass of solution = 1 kg = 1000 g
mass of solute = ?
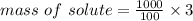
Mass of sugar = 30 g