Answer:
0.6 Molar
Step-by-step explanation:
According to the Question,
In 500 mL of water ,
0.2 mol sample of MgCl is present and 0.1 mol of KCl is present.
Thus ,
Concentration of Cl anion in the solution will be the sum of Cl anion concentration from MgCl and KCl.
From MgCl , 0.2 mol of Cl anion will come and 0.1 mol of KCl anion will come ,
Thus , 0.3 mol of Cl anion will come totally .
So, Cl anion concentration will be
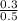 = 0.6 M
= 0.6 M