Answer:
-21 kJ·mol⁻¹
Step-by-step explanation:
Data:
H₃O⁺ + OH⁻ ⟶ 2H₂O
V/mL: 50 50
c/mol·dm⁻³: 1.0 1.0
ΔT = 4.5 °C
C = 4.184 J·°C⁻¹g⁻¹
C_cal = 50 J·°C⁻¹
Calculations:
(a) Moles of acid
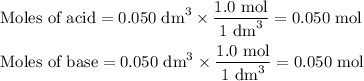
So, we have 0.050 mol of reaction
(b) Volume of solution
V = 50 dm³ + 50 dm³ = 100 dm³
(c) Mass of solution
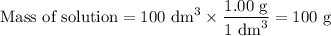
(d) Calorimetry
There are three energy flows in this reaction.
q₁ = heat from reaction
q₂ = heat to warm the water
q₃ = heat to warm the calorimeter
q₁ + q₂ + q₃ = 0
nΔH + mCΔT + C_calΔT = 0
0.050ΔH + 100×4.184×4.5 + 50×4.5 = 0
0.050ΔH + 1883 + 225 = 0
0.050ΔH + 2108 = 0
0.050ΔH = -2108
ΔH = -2108/0.0500
= -42 000 J/mol
= -42 kJ/mol
This is the heat of reaction for the formation of 2 mol of water
The heat of reaction for the formation of mol of water is -21 kJ·mol⁻¹.