Answer:
average pressure = 8.314 ×
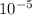 pascal
pascal
density = 1.66 ×
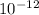 kg/m³
kg/m³
Step-by-step explanation:
given data
temperature of sun = 6000 K
concentration of atoms =
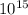 particles/m³
particles/m³
to find out
average pressure and density of its atmosphere
solution
first we get here average pressure of atmosphere that is express as
average pressure =
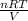 .............1
.............1
put here value we get
average pressure =
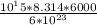
average pressure = 8.314 ×
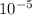 pascal
pascal
and
density of atmosphere will be
density =
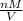 .............2
.............2
density =
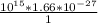
density = 1.66 ×
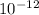 kg/m³
kg/m³