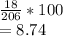Answer:
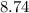 %
%
Step-by-step explanation:
Please see the complete question in the attachment
The given formula of Ibuprofen consists of 13 atoms of carbon, 18 atoms of hydrogen and 2 atoms of oxygen.
The atomic mass of carbon, hydrogen and oxygen is 12, 1 and 16 respectively.
The total atomic mass of Ibuprofen is equal to
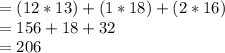
Percent composition of hydrogen by mass is equal to atomic mass of hydrogen divided by total mass of Ibuprofen.