Answer:
The boiling point of a 8.5 m solution of Mg3(PO4)2 in water is 394.91 K.
Explanation:
The formula for molal boiling Point elevation is :
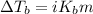
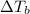 = elevation in boiling Point
= elevation in boiling Point
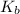 = Boiling point constant( ebullioscopic constant)
= Boiling point constant( ebullioscopic constant)
m = molality of the solution
i = Van't Hoff Factor
Van't Hoff Factor = It takes into accounts,The abnormal values of Temperature change due to association and dissociation .
In solution Mg3(PO4)2 dissociates as follow :
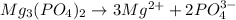
Total ions after dissociation in solution :
= 3 ions of Mg + 2 ions of phosphate
Total ions = 5
i = Van't Hoff Factor = 5
m = 8.5 m
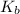 = 0.512 °C/m
= 0.512 °C/m
Insert the values and calculate temperature change:
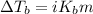
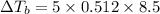
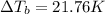
Boiling point of pure water = 100°C = 273.15 +100 = 373.15 K
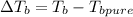
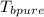 = 373.15 K[/tex]
= 373.15 K[/tex]
21.76 = T - 373.15
T = 373.15 + 21.76
T =394.91 K