Answer:
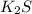
Step-by-step explanation:
According to the boiling point elevation law described by the equation
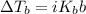 , the increase in boiling point is directly proportional to the van 't Hoff factor.
, the increase in boiling point is directly proportional to the van 't Hoff factor.
The van 't Hoff factor for nonelectrolytes is 1, while for ionic substances, it is equal to the number of moles of ions produced when 1 mole of salt dissolves.
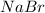 would produce 2 moles of ions per 1 mole of dissolved substance, sodium and bromide ions.
would produce 2 moles of ions per 1 mole of dissolved substance, sodium and bromide ions.
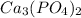 is insoluble in water, so it would barely dissociate and wouldn't practically change the boiling point.
is insoluble in water, so it would barely dissociate and wouldn't practically change the boiling point.
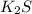 would dissociate into 3 moles of ions per 1 mole of substance, two potassium cations and one sulfide anion.
would dissociate into 3 moles of ions per 1 mole of substance, two potassium cations and one sulfide anion.
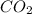 is a gas, it would form some amount of carbonic acid when dissolved, however, carbonic acid is molecular and would yield i value of i = 1.
is a gas, it would form some amount of carbonic acid when dissolved, however, carbonic acid is molecular and would yield i value of i = 1.
Therefore, potassium sulfide would raise a liquid's boiling point the most if all concentrations are equal.