Answer:
The molarity (M) of the following solutions are :
A. M = 0.88 M
B. M = 0.76 M
Step-by-step explanation:
A. Molarity (M) of 19.2 g of Al(OH)3 dissolved in water to make 280 mL of solution.
Molar mass of Al(OH)3 = Mass of Al + 3(mass of O + mass of H)
= 27 + 3(16 + 1)
= 27 + 3(17) = 27 + 51
= 78 g/mole
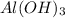 = 78 g/mole
= 78 g/mole
Given mass= 19.2 g/mole
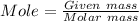
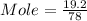
Moles = 0.246
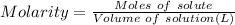
Volume = 280 mL = 0.280 L
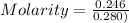
Molarity = 0.879 M
Molarity = 0.88 M
B .The molarity (M) of a 2.6 L solution made with 235.9 g of KBr
Molar mass of KBr = 119 g/mole
Given mass = 235.9 g
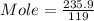
Moles = 1.98
Volume = 2.6 L
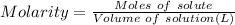
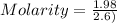
Molarity = 0.762 M
Molarity = 0.76 M