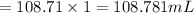Answer:108.71 mL
Step-by-step explanation:
Given
Volume of sample V=150 mL
concentration of sucrose solution 35 % w/w i.e. In 100 gm of sample 35 gm is sucrose
specific gravity =1.115
Density of solution
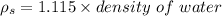
Thus
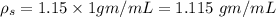
mass of sample
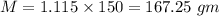
mass of sucrose
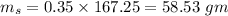
mass of Water
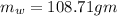
Volume of water