Answer : The volume of the stock solution he need to make the dilute solution will be, 45 mL
Explanation :
According to dilution dilution law:

where,
 = molarity of concentrated solution
= molarity of concentrated solution
 = molarity of dilute solution
= molarity of dilute solution
 = volume of concentrated solution
= volume of concentrated solution
 = volume of dilute solution
= volume of dilute solution
Given:
 =
=
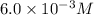
 =
=
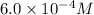
 = ?
= ?
 = 450 mL
= 450 mL
Now put all the given values in the above formula, we get:
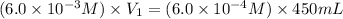
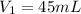
Therefore, the volume of the stock solution he need to make the dilute solution will be, 45 mL