Answer:
The grams of aluminum sulfide formed are ; 12.51 g
Step-by-step explanation:
Using Formula,
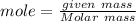
Calculate the moles of Al and S :
Given mass of Al = 9 g
Molar mass of Al = 27 g/ mole
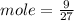
Mole of Al = 0.33
Given mass of S = 8 g
Molar mass of S = 32 g/mole
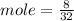
Mole of S = 0.25
The balanced chemical equation for the reaction between aluminium and sulfur is :
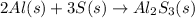
Here ,
2 mole of Al needs 3 moles of S
1 mole of Al needs 3/2(= 1.5) moles of S
hence ,
0.33 mole of Al should require
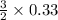 moles of S
moles of S
Sulfur needed = 0.495 mole
Available S = 0.25 mole
So there is less sulfur than required , S is the limiting reagent
Amount of S decide the Amount of Al2S3 formed
3 mole of S produce 1 mole of Al2S3
1 mole of S produce 1/3 mole of Al2S3
0.25 mole will produce :
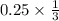
= 0.0833 moles of Al2S3
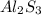 = 0.083 mole
= 0.083 mole
Molar mass of
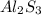 = 150.16 g/mole
= 150.16 g/mole
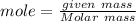
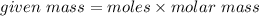
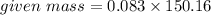
= 12.51 g