Answer:
correct option is a. 45,000 tons per day (equal to one large coal-fired power plant)
Step-by-step explanation:
solution
we know that Sulphur Dioxide
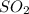 is more in amount when there is an explosive eruption
is more in amount when there is an explosive eruption
when Sulphur Dioxide
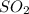 and carbon dioxide
and carbon dioxide
 near the surface they released in the atmosphere very rapidly so that the eruption is explosive
near the surface they released in the atmosphere very rapidly so that the eruption is explosive
and the Kilauea eruption was big and explosive it thrown out block and bombs etc
so here correct option is a. 45,000 tons per day (equal to one large coal-fired power plant)