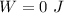Answer:
A.
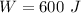
B.
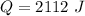
C.
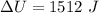
D.
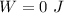
Step-by-step explanation:
Given:
- no. of moles of oxygen in the cylinder,
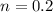
- initial pressure in the cylinder,
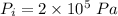
- initial temperature of the gas in the cylinder,
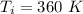
According to the question the final volume becomes twice of the initial volume.
Using ideal gas law:
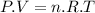
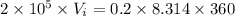
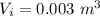
A.
Work done by the gas during the initial isobaric expansion:
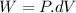
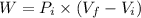
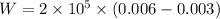
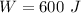
C.
we have the specific heat capacity of oxygen at constant pressure as:
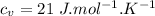
Now we apply Charles Law:
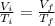
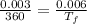
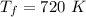
Now change in internal energy:
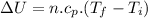
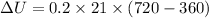
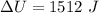
B.
Now heat added to the system:
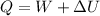
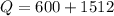
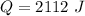
D.
Since during final cooling the process is isochoric (i.e. the volume does not changes). So,