Step-by-step explanation:
For what I can see, is missing the concentration of [Ag+] in the half-cell. To calculate it:
Niquel half-cell
Oxidation reaction:
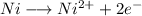
![E=E^0 - (R*T)/(n*F)*ln(1/[Ni^(2+)])](https://img.qammunity.org/2021/formulas/chemistry/high-school/9eho6vw9gw3ul52i49o6z4ykskj8hu1xd3.png)
Assuming T=298 K / R=8.314 J/mol K / F=96500 C
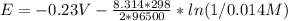
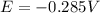
Silver half-cell
Reduction reaction:
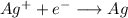
![E=E^0 - (R*T)/(n*F)*ln(1/[Ag+])](https://img.qammunity.org/2021/formulas/chemistry/high-school/pnxhoy1uldisjud5uo3j2qbswlomh7tjiv.png)
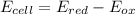
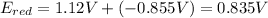
Assuming T=298 K / R=8.314 J/mol K / F=96500 C
![0.835V=0.8V - (8.314*298)/(1*96500)*ln(1/[Ag+])](https://img.qammunity.org/2021/formulas/chemistry/high-school/rlvm207q6fh9mh91dday58p726n7bp5wv4.png)
![[Ag+]=0.26 M](https://img.qammunity.org/2021/formulas/chemistry/high-school/uet3dw39abu25gz1gfhe2ahy6no8cqv8mi.png)