Answer:
a) 231.9 °C
b) 100% Sn
c) 327.5 °C
d) 100% Pb
Step-by-step explanation:
This is a mixture of two solids with different fusion point:
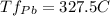
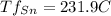
Given that Sn has a lower fusion temperature it will start to melt first at that temperature.
So the first liquid phase forms at 231.9 °C and because Pb starts melting at a higher temperature, that phase's composition will be 100% Sn.
The mixture will be completely melted when you are a the higher melting temperature of all components (in this case Pb), so it will all in liquid phase at 327.5 °C.
At that temperature all Sn was already in liquid state and, therefore, the last solid's composition will be 100% Pb.