Step-by-step explanation:
Formula for the prediction of sponteneity of a reaction is as follows:
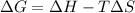
Where,
ΔG = Change in free energy
ΔH = Change in enthalpy
ΔS = Change in entropy
T = Temperature
If,
ΔG = -ve, then the reaction will be spontaneous
ΔG = 0, then the reaction will be in equilibrium
ΔG = +ve, then the reaction will be non-spontaneous