Answer:
0.91 atm is the partial pressure of just hydrogen gas.
Step-by-step explanation:
Vapor pressure of water , p= 0.0313 atm
Partial pressure of hydrogen gas =
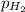
Total pressure of the water vapors and hydrogen gas = P = 715 Torr
1 atm = 715 Torr
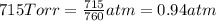
According Dalton's law of partial pressure:
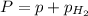
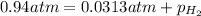
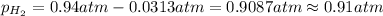
0.91 atm is the partial pressure of just hydrogen gas.