Answer:
Mass of aluminium in sample = 3.591 g ≅ 3.6 grams
Step-by-step explanation:
Given that, A sample of aluminum absorbs 50.1 J of heat, upon which the temperature of the sample increases from 20.0°C to 35.5°C.
the specific heat of aluminum is 0.900 J/g- °C
The relation between heat absorbed and change in temperature is given by, Q = msΔT.
where Q = heat absorbed
m = mass of the substance
s = specific heat of substance
ΔT = change in temperature
Now, in our case, Q = 50.1 J ; s = 0.900 J/g- °C; ΔT= 35.5-20 = 15.5°C
⇒ m =
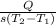
⇒ m =
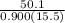 = 3.591 g ≅ 3.6 g
= 3.591 g ≅ 3.6 g
⇒ m ≅ 3.6 g