Answer:
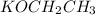 ,
,
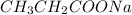 , NaOH are all ionic compounds.
, NaOH are all ionic compounds.
Step-by-step explanation:
It is known that ionic compounds are the compounds in which one atom transfer its valence electrons to another atom. Hence, during this transfer partial opposite charges tend to develop on the combing atoms due to which strong force of attraction exists between the atoms.
An ionic bond is always formed between a metal and a non-metal.
For example,
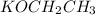 ,
,
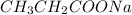 , NaOH are all ionic compounds.
, NaOH are all ionic compounds.
On the other hand, a compound formed due to sharing of electrons between the combining atoms is known as a covalent compound. Generally, a non-metal with same or different non-metal tends to form a covalent bond.
For example,
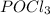 ,
,
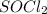 etc are all covalent compounds.
etc are all covalent compounds.
Thus, we can conclude that
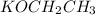 , [tex]CH_3CH_2COONa, NaOH are all ionic compounds.
, [tex]CH_3CH_2COONa, NaOH are all ionic compounds.