Answer: The volume of NaOH will be, 12.5 mL
Step-by-step explanation:
To calculate the volume of base, we use the equation given by neutralization reaction:
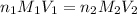
where,
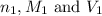 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is
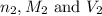 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.
We are given:
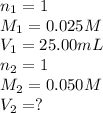
Putting values in above equation, we get:
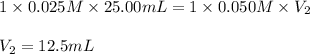
Hence, the volume of NaOH will be, 12.5 mL