Answer: Option (c) is the correct answer.
Step-by-step explanation:
Atomic number of sodium is 11 and its electronic configuration is
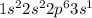 . When sodium loses one electron then it will attain +1 charge and its electronic configuration will be as follows.
. When sodium loses one electron then it will attain +1 charge and its electronic configuration will be as follows.
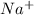 :
:
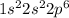
Atomic number of fluorine is 9 and its electronic configuration is
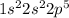 . When fluorine gains an electron then it acquires -1 charge and its electronic configuration is as follows.
. When fluorine gains an electron then it acquires -1 charge and its electronic configuration is as follows.
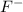 :
:
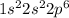
Atomic number of aluminium is 13 and its electronic configuration is
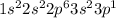 . When aluminium loses its valence electrons then it acquires +3 charge and its electronic configuration is as follows.
. When aluminium loses its valence electrons then it acquires +3 charge and its electronic configuration is as follows.
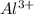 :
:
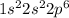
Thus, we can conclude that the listing for aluminum is correct.