Answer : The mass of sucrose added to water will be, 189.0 grams.
Explanation :
As we are given that 9 % solution (mass per volume) that means 9 grams of sucrose present in 100 mL volume of solution.
Total given volume of solution = 2.1 L = 2100 mL (1 L = 1000 mL)
Now we have to determine the mass of sucrose in solution.
As, 100 mL of solution contains 9 grams of sucrose
So, 2100 mL of solution contains
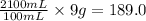 grams of sucrose
grams of sucrose
Therefore, the mass of sucrose added to water will be, 189.0 grams.