Answer:
a. Vₐ = 111.5282 + 1.29396m
b. For m = 0.100m; Vₐ = 111.6576
Step-by-step explanation:
The partial molar volume of compound A in a mixture of A and B is defined as :
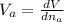
Where V is volume and n are moles of a.
a. As molality is proportional to moles of substance, partial molar volume of glucose can be defined as:
Vₐ = dV / dm = d(1001.93 + 111.5282m + 0.64698m²) / dm
Vₐ = 111.5282 + 1.29396m
b. Replacing for m = 0.100m:
Vₐ = 111.5282 + 1.29396×0.100
Vₐ = 111.6576
I hope it helps!