Answer:
The molecule having shortest C-C bond is acetylene (CHCH)
Step-by-step explanation:
Note : CH3CH2OH has longest bond length
The bond length order from longer to shorter is :
CH3CH2OH > CH3CH3 > CH2CH2 > CHCH
(More bond order shorter the bond length)
The C-C bond length in Ethanol
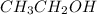 is more than ethane
is more than ethane
- Here there is single bond between the carbons
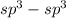 overlap
overlap
- It has lowest Bond-dissociation energy (88 Kcal/mole)
The C-C bond length in Ethane
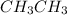 is 1.54 Angstrom
is 1.54 Angstrom
- Here there is single bond between the carbons
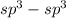 overlap
overlap
- It has low Bond-dissociation energy (90 kcal/mole : means bonds are long and broken easily but 2 kcal/mole stronger than ethane)
- Bonds are shorter than ethanol.
The C-C bond length in Ethene
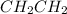 is 1.34 Angstrom
is 1.34 Angstrom
- Here there is double-bond between the carbons
- The C-C bond distance is shorter than ethane
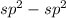 overlap
overlap
- It has high Bond-dissociation energy than ethane (bonds are stronger than ethane)
The C-C bond length in acetylene
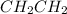 is 1.20 Angstrom
is 1.20 Angstrom
- Here there is triple-bond between the carbons
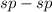 overlap
overlap
- The C-C bond distance is shortest of all
- It has maximum Bond-dissociation energy.Strongest and shortest bond