Answer: Option (B) is the correct answer.
Step-by-step explanation:
As we know that the temperature when the vapor pressure of liquid becomes equal to the atmospheric pressure surrounding the liquid. And, during this temperature liquid state of substance changes into vapor state.
But during this process of change in state of substance the temperature will cease to change for some time because unless and until all the liquid molecules do not convert into vapor state the temperature will not rise or change.
As the boiling point of water is
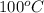 so the temperature ceases to change from
so the temperature ceases to change from
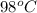 to
to
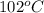 .
.
Therefore, we can conclude that when heating water, during
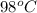 to
to
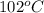 temperature range the temperature will cease to change for some time.
temperature range the temperature will cease to change for some time.