Answer:
Ionic bond
Step-by-step explanation:
Ionic bonding:-
This type of bonding is formed when there is a complete transfer of electrons from one element to another element. In this bonding one element is always a metal and another is a non-metal.
Thus, the atom which loses the electron which is gained by the another, there is a electrostatic attraction between two which which results in the formation of ionic bond.
For example:-
Calcium is the element of second group and forth period. The electronic configuration of Calcium is - 2, 8, 8, 2 or
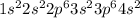
There are 2 valence electrons of Calcium.
Sulfur is the element of sixteenth group and third period. The electronic configuration of sulfur is - 2, 8, 6 or
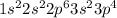
There are 6 valence electrons of sulfur.
Thus, calcium loses two electrons to sulfur and sulfur accepts these electrons to form ionic bond.
Calcium sulfide,
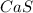 is formed when 2 valence electrons of calcium are loosed and they are gained by sulfur atom.
is formed when 2 valence electrons of calcium are loosed and they are gained by sulfur atom.