Answer:
The difference in mass between 3.01×10^24 atoms of gold and a gold bar with the dimensions 6.00 cm X 4.25 cm X 2.00 cm is :
Difference in mass = 985.32 - 984.5 = 0.82 g
Step-by-step explanation:
Part I :
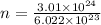
n = 4.9983
n = 4.99 moles
(Note : You can also take n = 5 mole )
Molar mass of gold = 196.96 g/mole
This means, 1 mole of gold(Au) contain = 196.96 grams
So, 4.99 moles of gold contain =
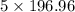 g
g
4.99 moles of gold contain = 984.8 g
Mass of
 atoms of gold = 984.5 g
atoms of gold = 984.5 g
Part II :
Density of Gold =
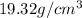
Volume of the cuboid =
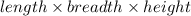
Volume of the gold bar =
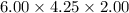
Volume of the gold bar = 51
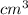
Using formula,
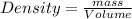
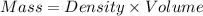
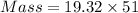
Mass = 985.32 g
So, A gold bar with the dimensions 6.00 cm X 4.25 cm X 2.00 cm has mass of 985.32 g
Difference in mass = 985.32 - 984.5 = 0.82 g