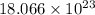Answer:
The number of chlorine atoms present in
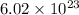 units of gold III chloride is
units of gold III chloride is
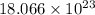
Step-by-step explanation:
Formula of Gold (III) chloride:
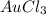
Avogadro Number : Number of particles present in one mole of a substance.
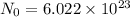
Using,
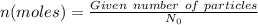
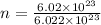
= 1 mole(0.9999 , nearly equal to 1 )
The given Gold III chloride sample is 1 mole in amount.
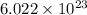 = 1 mole of
= 1 mole of
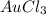
In this Sample,
1 mole of
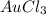 will give = 3 mole of Chlorine atoms
will give = 3 mole of Chlorine atoms
1 mole of Cl contain =
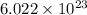
3 mole of Cl contain =
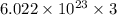
3 mole of Cl contain =
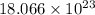
So,
The number of chlorine atoms present in
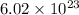 units of gold III chloride is
units of gold III chloride is