Answer:
38.38 L (3 d.p.)
Step-by-step explanation:
In response to questions addressing the expansion of gas via heating, Charles's Law should be considered. Charles's Law is an experimental gas law explaining the tendency of gases to expand when heated.
The law states that the volume (
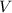 ) of a gas is directly proportional to its temperature (
) of a gas is directly proportional to its temperature (
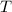 ) given that the pressure (
) given that the pressure (
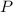 ) and the amount of gas (
) and the amount of gas (
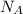 ) are constant.
) are constant.
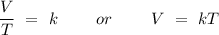 , where k is a proportionality constant.
, where k is a proportionality constant.
*Note that when substituting temperature into the equation, the temperature must be in terms of the Kelvin scale and not in degrees Celsius. The relation between the Kelvin scale and the Celsius scale is
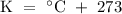 .
.
Charles's law can be used to compare changing conditions of a particular gas. Let
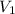 and
and
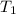 be the initial volume and temperature for the gas, while
be the initial volume and temperature for the gas, while
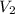 and
and
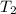 be the final volume and temperature. The mathematical relationship of Charles's law becomes:
be the final volume and temperature. The mathematical relationship of Charles's law becomes:
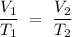 .
.
In accordance with the question,
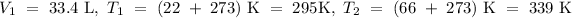
while
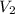 is the final volume of the gas that we would like to find. Therefore,
is the final volume of the gas that we would like to find. Therefore,
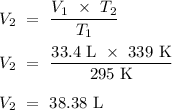 .
.