The mass of water required will be approximately
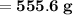 .
.
- To solve this question, just make a simple rule of three between the amount of salt dissolved at 80ºC and the mass of water:
 45g of salt
45g of salt
 100g of H₂O
100g of H₂O
 250g of salt
250g of salt
 x g of H₂O
x g of H₂O

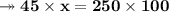

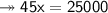

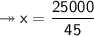

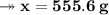
- Therefore, knowing that the solubility of a salt at 80ºC is 45g/100g of water (H₂O), the mass of water needed to dissolve 250g of this salt at 80ºC will be
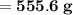
__________________________________________________