Answer:
So we're trying to find the number of carbon moles in the compound. We know that we have 200g of the compound and that 40% of it is Carbon. and 40% of 200 is 80g (change 40% into a decimal and multiply it by 200 and you get 80g)
So 80g of the compound is carbon. In order to find the moles of Carbon, I have to divide the mass by the molar mass of the element.
We know from our periodic table that the molar mass for carbon is 12g/mol
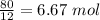
Therefore we have 6.67 moles of carbon in the compound