Answer:
TiCl₄
Step-by-step explanation:
From the law of conservation of mass, the mass of the reactants must equal the mass of the products. In other words:
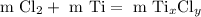
Find the mass of chlorine gas reacted:
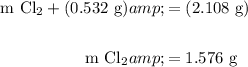
Find the number of moles of each reactant using their respective molecular weights:
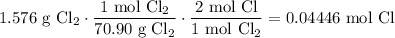
And:
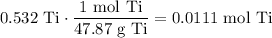
To find the empirical formula, all values by the smallest value:
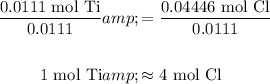
Hence, there are four moles of chlorine for every one mole of titanium.
In conclusion, our empirical formula is TiCl₄.