Answer:
Take 49.2-ml of 18.0 M H2SO4 stock solution and pour it into a 500-ml volumetric flask. Fill to the 500-ml line with distilled water to make 1.77M H2SO4 solution.
Step-by-step explanation:
use the formula M1V1=M2V2
volume must be in liters(L)
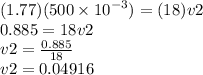
0.04916L × 1000 = 49.16 ml = 49.2 ml