Answer:
Step-by-step explanation:
Hey there!
From question;
The molecular mass of NH₄OH is 35.
Avogadro number = 6.023*10²³ molecules.
Then,
From mole concept,
Molecular mass = Avogadro number
So, 35 grams contains 6.023*10²³ molecules.
1 gram contains
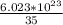 molecules.
molecules.
52.1 grams contains
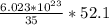 molecules.
molecules.
Therefore, 52.1 grams contains 8.96*10²³ molecules.
Hope it helps!