Answer:
C
Step-by-step explanation:
We want to calculate the volume of carbon dioxide gas produced at STP upon the decomposition of 0.150 g of CaCO₃ illustrated by the equation:
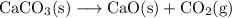
To do so, we can convert from grams of CaCO₃ to moles of CaCO₃; moles of CaCO₃ to moles of CO₂; and moles of CO₂ to liters of CO₂ (at STP).
Find the molecular weight of CaCO₃:
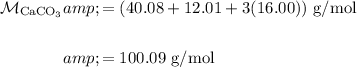
From the equation, one mole of CO₂ is produced from every one mole of CaCO₃.
Finally, recall that at STP, one mole of any gas occupies a volume of 22.4 L.
Hence:
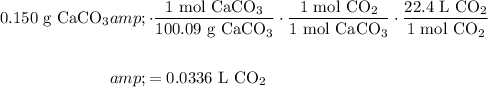
In conclusion, about 0.0336 liters of carbon dioxide is produced.
Our answer is C.